Abstract
Background
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease in adult patients with respect to clinical presentation, histopathology and molecular-genetic features. While the long-term cure rate after rituximab-containing conventional chemotherapy programs exceeds 80% in young patients with low and low-intermediate risk according to International Prognostic Index (IPI), similar treatments still remain unsatisfactory in patients belonging to high and high-intermediate risk (Pfreundschuh M Lancet Oncol 12:1013-1022, 2011; Coiffier B Blood 116:2040-2045, 2010; Pfreundschuh M Lancet Oncol 7: 379-391, 2006).
For these patients the optimization of front-line therapy remains an important objective. Advances in molecular genetics have vastly improved the understanding of the biological diversity in DLBCLs, leading to the discovery of key oncogenic pathways and novel therapeutic targets.
In a recently published randomized phase III trial we recruited 246 untreated DLBCL patients with a high-intermediate (56%) or high (44%) IPI score, in order to compare the R-CHOP-14 to the R-HDS plus ASCT therapeutic protocol. In this study the definition of the cell of origin (COO) by immunohistochemistry was unable to discriminate the clinical outcome according to the allocated treatment (Cortelazzo S J Clin Oncol. 2016 33:4015-4022). Herein, we show that a molecular classification of DLBCL may lead to identify patients who most likely benefit from treatment intensification.
Methods and Patients
We were able to run digital multiplex gene expression profiling (Nanostring technology) in 62 of the 246 patients, for whom formalin-fixed paraffin-embedded (FFPE) material was available, to classify them into the molecular COO types: ABC (Activated B Cell), GCB (Germinal Center B Cell) or Unclassified.
The Nanostring panel contains 28 genes: 20 genes are used to assign COO subtype according to LST algorithm (Scott D Blood 123: 1214-1217, 2014), the remaining 8 genes are employed to assess MYC and BCL2 expression, to quantify TP53 level, to detect potential therapeutic targets (STAT3, NFKB, PTEN and PKI3CA) and to evaluate macrophages infiltrate (CD68).
Results
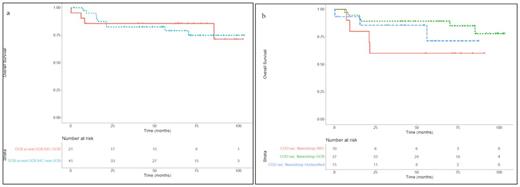
First of all, we confirmed that the distinction of DLBCL into COO categories by gene expression was more accurate than immunohistochemistry (IHC) determination, which failed especially in classifying non-GCB cases (86% of concordance in GCB cases, 53,6% in ABC plus Unclassified group, 22% considering only ABC). Indeed, considering the entire cohort, the Overall Survival (OS) was significantly different comparing GCB to ABC patients, while IHC failed to identify prognostic subgroups (Figure 1a and 1b).
Regarding the effects of the two therapeutic treatments on OS, when we analyzed the 62 patients together or only the GCB group, we observed no significant difference between the two arms. Instead, in ABC group we noticed a clear difference in OS between R-HDS, with 83% of cases that survived, and R-CHOP14, after which only 25% of cases survived.
Furthermore we investigated a possible correlation between MYC and BCL2 IHC and Nanostring mRNA expression data, we found no linear relationship but we noticed a positive trend. Moreover BCL2 and MYC expression showed a higher predictive OS value than IHC.
Conclusions
Our results suggest that the diagnostic FFPE gene expression assay by Nanostring robustly identifies DLBCL subgroups according to the COO. Moreover, yet preliminary, the molecular definition of the COO is able to identify patients at high risk of poor outcome when treated with R-CHOP and who may benefit by intensified high dose chemotherapy programs or experimental new treatments.
Rambaldi: Novartis, Amgen, Celgene, Sanofi: Other: Travel, Accomodations, Expenses; Novartis, Roche/Genentech, Amgen, Italfarmaco: Consultancy. Cesano: NanoString Technologies: Employment, Equity Ownership. Pileri: Takeda Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal